Hydrogen Peroxide Test Kits
What is Hydrogen Peroxide?
Peroxides are chemicals with an oxygen to oxygen bond. Hydrogen peroxide (H2O2) is the simplest peroxide. It is a colorless or pale blue liquid at room temperature, depending on concentration, that breaks down into the by-products of water and oxygen. Hydrogen peroxide finds use in many different industries due to its strong oxidizing properties. For the pulp and paper industry, it is used as a bleaching agent. In municipal drinking water, wastewater and water treatment industries, H2O2 is utilized to control taste and odor, and to remove hydrogen sulfide and iron. Various forms of advanced oxidation processes rely on H2O2 to effectively remediate environmental contamination. Hot hydrogen peroxide mists are used as a sterilant in aseptic and extended shelf-life packaging (ESL) equipment as the packages are formed in line.
Why Test for Hydrogen Peroxide?
Hydrogen peroxide treatment in food packaging must eliminate microbial pathogenic contamination. However, hydrogen peroxide residuals must then be removed before the filling process to prevent negative impacts to the products’ taste and nutritional profiles. The US FDA specifies a limit on H2O2 residual in packaging of no more than 0.5 parts per million (ppm). This hydrogen peroxide level is often referenced globally as an acceptable residual limit.
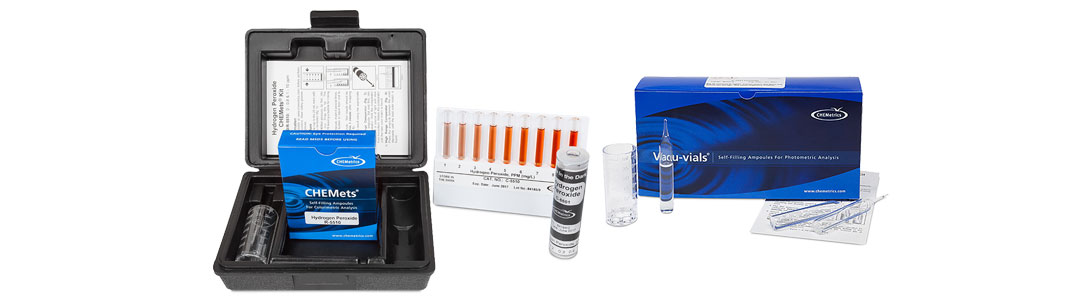
About Our Test Kits
CHEMetrics offers a variety of visual kits for measuring the concentration of hydrogen peroxide in water. CHEMets® test kit K-5502 utilizes the DPD chemistry to measure hydrogen peroxide from 0 to 0.5 ppm. Several visual kits that employ the ferric thiocyanate chemistry are available for determination of hydrogen peroxide at low and high concentration ranges. We also offer a Titrets® test (Cat. No. K-5530) that uses a ceric sulfate titrant with ferroin indicator. Depending on the procedure used, this kit can determine H2O2 concentrations from 0.01% to as high as 20%.
For instrumental testing, our Vacu-vials® ampoules (Cat. No. K-5543) utilize the ferric thiocyanate chemistry. Instrumental kits require the use of CHEMetrics direct-readout photometers or spectrophotometers capable of accepting a 13mm diameter round cell.
CHEMetrics also offers a 0.5 ppm hydrogen peroxide analytical standard (Cat. No. A-5505) that can be used to verify kit reagent and instrument performance reliability.
Further Reading
» Hydrogen Peroxide Analysis in the Presence of Peracetic Acid and Copper
» Hydrogen Peroxide Comparative Performance Study
Click on a catalog number in the tables below for more information on how to test for hydrogen peroxide or to purchase a test kit.
Visual Kits
| Range | MDL | Method | Kit Catalog No. | Refill Catalog No. |
|---|---|---|---|---|
| 0-0.5 ppm | 0.025 ppm | DPD | K-5502 | R-7904 |
| 0-0.8 & 1-10 ppm | 0.05 ppm | Ferric Thiocyanate | K-5510 | R-5510 |
| 0-25 & 30-300 ppm | 5 ppm | Ferric Thiocyanate | K-5510D | R-5510D |
| 0-50 & 60-600 ppm | 10 ppm | Ferric Thiocyanate | K-5510A | R-5510A |
| 0-100 & 120-1200 ppm | 20 ppm | Ferric Thiocyanate | K-5510B | R-5510B |
| 0-1000 & 1200-12,000 ppm | 200 ppm | Ferric Thiocyanate | K-5510C | R-5510C |
| 0.1-1.0% (up to 20% with dilution) | 0.10% | Ceric Sulfate Titrant with Ferroin Indicator | K-5530 |
Instrumental Kits
| Range | Method | Kit Catalog No. |
|---|---|---|
| 0-6.00 ppm | Ferric Thiocyanate | K-5543 |
| 0-6.00 ppm | Ferric Thiocyanate | I-2016 |
Analytical Standard
| Product | Kit Catalog No. |
|---|---|
| Hydrogen Peroxide Standard | A-5505 |
Methods
The Ferric Thiocyanate Method
Reference: APHA Standard Methods Online, Method 4500-H2O2 B-2020.
The ferric thiocyanate method consists of ammonium thiocyanate and ferrous iron in acid solution. Hydrogen peroxide oxidizes ferrous iron to the ferric state, resulting in the formation of a red thiocyanate complex. Chlorine will not interfere with this method. Ferric iron, peracetic acid, and cupric copper will interfere. Results are expressed as ppm (mg/L) H2O2.
The DPD Method
References: USEPA Methods for Chemical Analysis of Waters and Wastes, Method 330.5 (1983). APHA Standard Methods, 23rd ed., Method 4500-Cl G-2000. D.F. Boltz and J.A. Howell, eds., Colorimetric Determination of Nonmetals 2nd ed., Vol. 8, p. 303 (1978).
With the DPD Method, hydrogen peroxide reacts with DPD (N, N-diethyl-p-phenylenediamine) in the presence of potassium iodide and ammonium molybdate to form a pink color. Various oxidizing agents such as halogens, ozone, and peracetic acid will produce high test results. Results are expressed as ppm (mg/L) H2O2.
The Ceric Sulfate Titrimetric Method
Reference: Developed by CHEMetrics, LLC
CHEMetrics developed a titrimetric method using ceric sulfate as the titrant and ferroin as the end point indicator. A color change from green to orange signals the end of the titration. Results are expressed as percent (%) H2O2. The test range can be modified by performing a sample dilution. Details are provided in the kit instructions for ranges of 0.01 – 0.1% through 2- 20%.


