Food and beverage packaging takes many shapes and forms in today’s marketplace. In an effort to maintain grocery shelf-life and flavor quality, these products are processed and assembled by sophisticated packaging equipment that has evolved away from traditional bottling and canning methods.
Systems of quality control in the food and beverage industry have become more sophisticated as well as companies have come under the FDA’s Hazard Analysis and Critical Control Points (HACCP) guidelines. They call for food companies to develop quality monitoring procedures at critical control production points. Furthermore, corrective actions must be identified and taken if established limits are not met. Lastly, effective recordkeeping of food safety monitoring must be maintained to document the various HACCP-mandated processes.
Aseptic Packaging
Aseptic packaging technology utilize both hydrogen peroxide and heat to achieve sterility enabling food products to be distributed through ambient temperature channels. FDA approved these processes in January 1981 in response to a petition by Tetra Pak. Within months, some of the largest U.S. beverage producers (juice, dairy, etc.) began employing aseptic packaging sterilization procedures. Although new to the U.S. at that time, the technology’s origin can be traced to Sweden’s Tetra Brik packaging introduced in 1963.

Extended Shelf Life
The chilled food and beverage segment of the market has boomed since the 1980s as packaging engineers have employed variations on the “aseptic theme” to produce systems that prolong shelf life beyond that of traditional pasteurized products (hence extended shelf-life or ESL). Although ESL processes apply a heat/time regimen to the product that is regarded as a sterilization process, ESL packaging operations do not necessarily sterilize the packages or package enclosures, therefore all ESL products are distributed through refrigerated channels. Examples of ESL products offered in the refrigerated section of the grocery store are orange juice (not from concentrate), flavored milk, coffee creamers, and puddings.
General Protocols Used in Packaging Systems
Regardless of the food packaging system being considered, packaging vendors apply the same basic microbiological and engineering principles to design their food safety monitoring equipment. Precise details of the processes are dictated by the product type – high acid or low acid (aka pH). The general procedures used to sterilize either the product or packaging systems before any product or package enters the system involve:
- steam
- steam plus hydrogen peroxide
- hydrogen peroxide
- peracetic acid
- other chemical treatments, or
- hot water.
When chemical sterilization is applied to package interiors or closures, residual chemical must be removed prior to filling not only to comply with FDA residual regulations but also to maintain sensory quality and prevent flavor degradation.
Hydrogen Peroxide Measurement, Monitoring and Control
In 21 CFR 178.1005 (a) of the Code of Federal Regulations, hydrogen peroxide is defined to be a 35% aqueous solution. In subsection (d) of this same standard, it specifies limits on the hydrogen peroxide residual. “No use of hydrogen peroxide solution in the sterilization of food packaging material shall be considered to be in compliance if more than 0.5 part per million of hydrogen peroxide can be determined in distilled water packaged under production conditions (assay to be performed immediately after packaging).”

Analytical Tools for the Detection of Hydrogen Peroxide
The traditional laboratory bench method used to determine hydrogen peroxide levels is a titration with potassium permanganate (KMnO4). This requires volumetric glassware, use of buret, and standardization of the KMnO4 prior to testing. Typically, titrations are repeated up to three times to determine an averaged test result. In the manufacturing arena, where it is not uncommon for lines to produce hundreds of bottles per minute, waiting for a lab result to confirm residuals is costly.
Paper hydrogen peroxide test strips offer advantages over titrimetric methods. Typically, a strip is dipped in a sample for a specified time, removed and allowed to stand while a color reaction develops on the reagent pad. The developed color is then matched to a printed color standard. One disadvantage of paper test strips is that they are deactivated by moisture. Care must therefore be taken to prevent exposure of the strips to air.
Even under ideal conditions, hydrogen peroxide test strips have a limited shelf-life. Furthermore, test strips may not offer the sub-ppm sensitivity required for residual testing. Lastly, test results may be influenced by user technique – for example, how vigorously the strip is stirred in the sample, and the degree to which the sample is allowed to drain from the strip once the strip is removed from it.
About CHEMetrics
CHEMetrics manufactures an innovative, colorimetric hydrogen peroxide test kit that is economically priced and offers:
- immediate test results (in less than 2 minutes) at the point of testing, not in the lab
- long term reagent stability
- sub ppm sensitivity, and
- accuracy independent of user technique.
In this analytical system, the hydrogen peroxide liquid reagent is pre-dosed and packaged in a vacuum-sealed ampoule. In the visual test kit, the CHEMets® ampoule tip is immersed in the sample, the tip is snapped off, and the sample is automatically drawn into the ampoule. After the ampoule is inverted several times to facilitate mixing, it is compared to color standard ampoules to obtain a test result. An instrumental version of this hydrogen peroxide test kit is also available. The same test procedure is followed except that the ampoule (Vacu-vials®) is read in a photometer rather than compared visually to color standards.
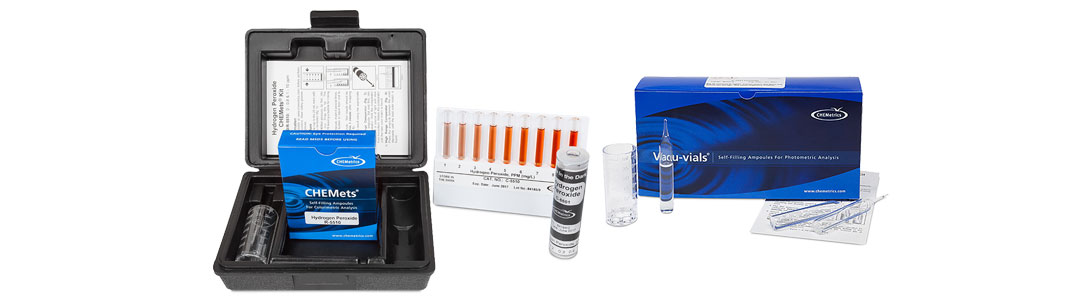
CHEMetrics offers a variety of methods and test kit configurations that permit the measurement of hydrogen peroxide concentrations ranging from sub ppm to percent levels. The analytical method that is most widely used by food and beverage customers for hydrogen peroxide food safety is the ferric thiocyanate chemistry. In this chemistry, the ampoules contain a ferrous ammonium thiocyanate reagent. Hydrogen peroxide in the sample converts ferrous ammonium thiocyanate to ferric thiocyanate. The intensity of the orange-brown colored ferric thiocyanate is proportional to the hydrogen peroxide level in the sample. Test results are obtained in two minutes or less. The measurement range for the ferric thiocyanate CHEMets® test kit (visual) is 0 – 0.8 ppm and 1 – 10 ppm, with a detection limit of 0.05 ppm. The measurement range for the ferric thiocyanate Vacu-vials® test kit (instrumental) is 0.15 – 6.00 ppm, with a detection limit of 0.15 ppm. VACUettes® kits are also available in which the ampoules have been fitted with an auto-dilutor tip, thus allowing for measurement up to 1.2% (12,000 ppm) hydrogen peroxide.
Other CHEMetrics kit options available for measuring high levels of hydrogen peroxide (up to 20%) employ a titrimetric ceric sulfate reagent and a ferroin indicator. Titrets® ampoules employ a reverse titration method that employs pre-dosed, vacuum-sealed reagent. The sample is drawn into the ampoule in small doses until a sharp endpoint color change signals the equivalence point has been reached. Quantitative test results are read directly from a scale printed on the side of the Titrets® ampoule.
Lastly, a ceric sulfate Go-No-Go test kit format is available upon request for situations where a Pass/Fail result at a specified control point is sufficient. A single, small dose of sample is added to a screw cap vial containing the hydrogen peroxide liquid reagent and endpoint indicator. An immediate color change occurs to signal that the hydrogen peroxide level in the sample is either above or below the specified control point.
Food processors must weigh analysis cost, turn-around time, accuracy, sensitivity and ease of use when determining which analytical sterilization test method suits their requirements. CHEMetrics hydrogen peroxide test kits, with their “snap and read” approach to sample analysis, fulfill each of these requirements with distinction.

