Iron Test Kits
What is Iron?
Iron is a metal that is present in nature in the form of its oxides, or in combination with silicon or sulfur. In natural environments iron dissolves into water when it runs over rocks or soil that contains iron. The soluble iron content of surface waters rarely exceeds 1 mg/L, while ground waters often contain higher concentrations. The National Secondary Drinking Water Standard for iron is 0.3 mg/L, as iron concentrations in excess of 0.3 mg/L impart a foul taste and cause staining. High concentrations in surface waters can indicate the presence of industrial effluents or runoff.
Iron is mainly present in two forms: the soluble ferrous iron or the insoluble ferric iron. Water that contains ferrous iron is clear and colorless as the iron is totally dissolved into the water. When the iron is oxidized it turns into ferric iron. Water containing ferric iron has a reddish-brown color and possibly a sediment that will not dissolve.
Why Test for Iron?
Iron is not hazardous to health, but it is an aesthetic contaminant. Ferrous iron gives water a metallic taste and when used in drinks it turns an inky black color and cause discoloration to food. Iron can also leave ugly reddish-brown stains on fixtures and fabrics that it encounters. Iron can also build up inside pipes causing damage or reduced flow rate.
Using iron test kits, Many residences that use well water monitor iron concentration to prevent these problems.
The drinking water industry measures iron to maintain water quality. Laundromats also ensure that their system is iron-free to prevent staining. Boilers and power plants monitor iron concentration to prevent corrosion and maintain efficient heat exchange.
Iron is a key parameter for the oil and gas industry. Iron contamination in oil field brines are typically the result of corrosion processes. Accumulation of insoluble iron salts in a brine completion fluid can result in substantial formation damage and significantly affect an oil well’s productivity. Quantifying total iron in brine is critical to prevent costly shutdowns and maintenance.
About Our Test Kits
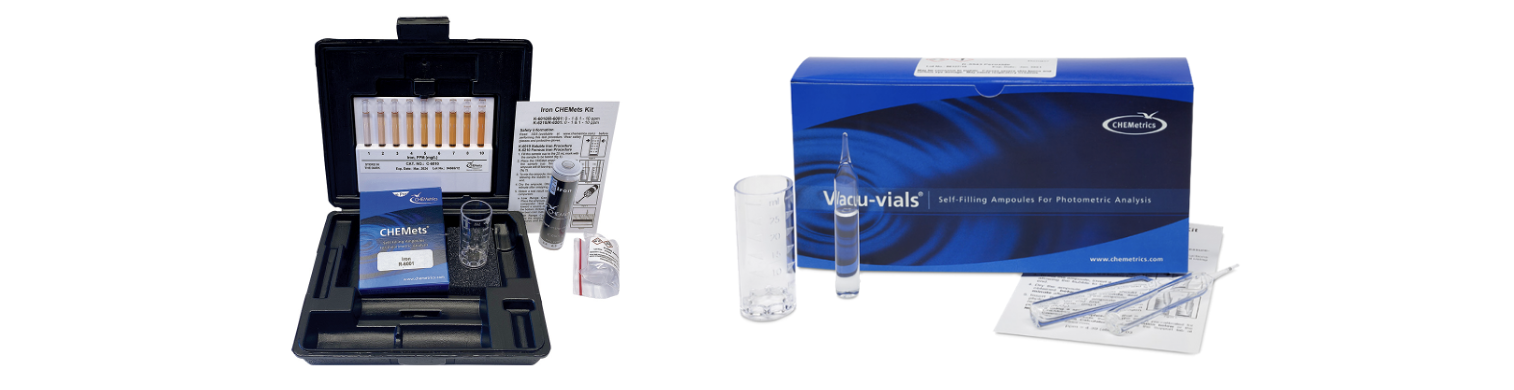
CHEMetrics visual iron testing kits feature CHEMets® ampoules. CHEMets ampoules are self-filling and contain pre-measured reagent for a single test. Simply snap the ampoule directly in a sample to draw in the correct volume of sample and then compare to color standards to find the concentration.
CHEMetrics offers both visual and instrumental iron test kits for measuring the concentration of iron in its different forms.
K-6210(D) are visual test kits utilizing the phenanthroline method to measure total & ferrous iron.
K-6010(A-D) are visual test kits utilizing hydroxylamine with the phenanthroline chemistry to measure total & soluble iron.
K-6002 is a visual test kit utilizing the ferric thiocyanate method to measure iron in brine.
CHEMetrics instrumental iron testing kits for water feature Vacu-vials® ampoule technology. Vacu-vials ampoules utilize the same self-filling technology as CHEMets ampoules, but the vials have a 13mm diameter, so they are compatible with most photometers or spectrophotometers.
K-6203 utilizes the phenanthroline method to measure total & ferrous iron.
K-6003 utilizes hydroxylamine with the phenanthroline chemistry to measure total & soluble iron .
Click on a catalog number in the tables below for more information or to purchase an iron testing kit.
Visual Kits
| Range | MDL | Method | Kit Catalog No. | Refill Catalog No. |
|---|---|---|---|---|
| 0-1 & 1-10 ppm | 0.05 ppm | Phenanthroline (total & ferrous) | K-6210 | R-6201 |
| 0-1 & 1-10 ppm | 0.05 ppm | Phenanthroline (total & soluble) | K-6010 | R-6001 |
| 0-30 & 30-300 ppm | 5 ppm | Phenanthroline (total & ferrous) | K-6210D | R-6201D |
| 0-30 & 30-300 ppm | 5 ppm | Phenanthroline (total & soluble) | K-6010D | R-6001D |
| 0-60 & 60-600 ppm | 10 ppm | Phenanthroline (total & soluble) | K-6010A | R-6001A |
| 0-100 & 100-1000 mg/L | 5 mg/L | Ferric Thiocyanate (Iron in brine) | K-6002 | R-6002 |
Instrumental Kits
| Range | Method | Kit Catalog No. |
|---|---|---|
| 0-6.00 ppm | Phenanthroline (total & ferrous) | K-6203 |
| 0-6.00 ppm | Phenanthroline (total & soluble) | K-6003 |
Methods
References: APHA Standard Methods, 23rd ed., Method 3500-Fe B – 1997. ASTM D 1068-77, Iron in Water, Test Method A. J.A. Tetlow and A.L. Wilson, “The Absorptiometric Determination of Iron in Boiler Feed-water,” Analyst. Vol. 89, p. 442 (1964).With the Phenanthroline method, ferrous iron reacts with 1,10-phenanthroline to form an orange-colored chelate. To determine total iron, thioglycolic acid solution is added to reduce ferric iron to the ferrous state. The reagent formulation minimizes interferences from various metals. Results are expressed as ppm (mg/L) Fe.
Reference: D. F. Boltz and J. A. Howell, eds., Colorimetric Determination of Nonmetals, 2nd ed., Vol. 8, p. 304 (1978). Carpenter, J.F. “A New Field Method for Determining the Levels of Iron Contamination in Oilfield Completion Brine”, SPE International Symposium (2004).
The Iron in Brine test employs the ferric thiocyanate chemistry. In an acidic solution, hydrogen peroxide oxidizes ferrous iron. The resulting ferric iron reacts with ammonium thiocyanate forming a red-orange colored thiocyanate complex, in direct proportion to the iron concentration.
Results, expressed in mg/L, can be converted to mg/kg by dividing by the density of the brine.

