What is a Research Paper Summary?
At CHEMetrics we love learning about how scientists use our test kits in the course of their research endeavors. To that that end, we keep our ears tuned for reports in the chemistry, medical, environmental and engineering oriented literature to find real world examples of how data generated from our test kits support recommendations for adapting or changing existing processes in order to improve desired outcomes. We will periodically highlight an example of these research topics to illustrate the wide range of scientific applications to which CHEMetrics test kits find use. The web link to the original publication will be provided at the end of the post for readers who wish to dig deeper into the subject of peracetic acid air monitoring.
Properties and Uses of Peracetic Acid
Peracetic acid (PAA) is a versatile oxidizing disinfectant that is formed from a reaction between acetic acid and hydrogen peroxide. It is commonly used as a sanitizing agent in the poultry processing and beverage industries to disinfect carcasses, equipment, pasteurizers, tanks, pipelines, evaporators, fillers, and contact surfaces. The pulp and paper industry uses peracetic acid as a delignification and bleaching agent. PAA is also finding use as a biocide in wastewater treatment and is even used in aquaculture tanks. Part of PAA’s rising popularity is that it decomposes into non-toxic by-products. However, since PAA is irritating to mucous membranes of the respiratory tract, skin, and eyes, occupational health professionals must consider the effect of worker exposure to PAA vapors. Consequently, there is a need to quantify and monitor air concentrations of PAA quickly and accurately in the workplace to compare against concentration thresholds that have been established for irritation to the upper respiratory tract and lacrimation.
Measuring Peracetic Acid Vapor Using Impingers
Recently an article published in the Journal of Occupational and Environmental Hygiene reported on a new method for measuring peracetic acid in air. The article, co-written by three authors from the National Institute for Occupational Safety and Health, is titled “A field-portable colorimetric method for the measurement of peracetic acid vapors: a comparison of glass and plastic impingers.” An impinger, a device designed to sample and collect airborne contaminants, consists of an inlet that is connected to a calibrated pump. The inlet is also connected to a receiving tube or nozzle which is submerged in a known volume of water that functions as a trap for the contaminant. The air is bubbled through the water. The pump is run for a designated collection period. The volume of air sampled (often expressed as cubic meters) can be calculated from the air flow per minute and the collection time. When the water in the trap is analyzed for the contaminant concentration, estimates of the mass of contaminant per cubic meter of air can be made (mg/m3).
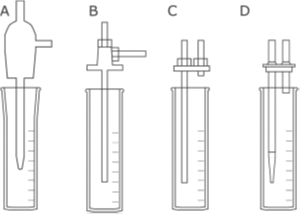
Using CHEMetrics Vacu-vials® Ampoules In Research
The researchers used CHEMetrics K-7913 PAA Vacu-vials test kit and V-2000 multi-analyte photometer to validate the performance of four impinger designs. To do this, they transferred a precise volume of PAA solution of known concentration to an Acrodisc syringe filter which was connected to the impinger inlet. Then a vacuum pump was turned on for 15 minutes. After the pump was turned off, the water in the trap was diluted and measured with K-7913 and V-2000 Program No. 148.

Figure 1 provides a graphic of the different types of impingers in this study. One of the impingers was a traditional glass impinger while the other three were made of plastic. Two of the commercially available plastic designs differed in the inlet and outlet impinger orientation (vertical versus horizontal), while the third plastic impinger replicated the horizontal outlet impinger but was differentiated by the nozzle design. In this case, the nozzle was 3D printed and changed from a blunt end to a tapered end. The researchers demonstrated that a tapered nozzle, (Fig. 1 A and D), transfers PAA vapor more efficiently than the blunt or flat ended nozzles, (Fig. 1 B and C).
The researchers also used the CHEMetrics Hydrogen Peroxide Vacu-vials Instrumental Kit (K-5543) to confirm that hydrogen peroxide did not significantly interfere with PAA measurement using CHEMetrics K-7913 PAA test kit. This was a significant finding because other NIOSH colorimetric PAA air sampling methods require the use of a filter cassette to remove hydrogen peroxide.
Validating the performance of a plastic impinger is one of the key results from this study since glass impingers are not always permitted in pharmaceutical and food and beverage facilities. Lastly the researchers were able to establish limit of detection and limit of quantitation values that were below the American Conference of Governmental and Industrial Hygienists’ threshold limit value for short-term exposure (ACGIH TLV STEL).
Results of the Experiment
The authors concluded the air sampling and measurement method could be used in the field to monitor PAA as part of a measurement strategy to evaluate worker exposure. The test method offers a low-cost alternative that provides a quick analysis turnaround time. They plan to publish their method in the NIOSH Methods Manual soon.
A link to the abstract is available Here

